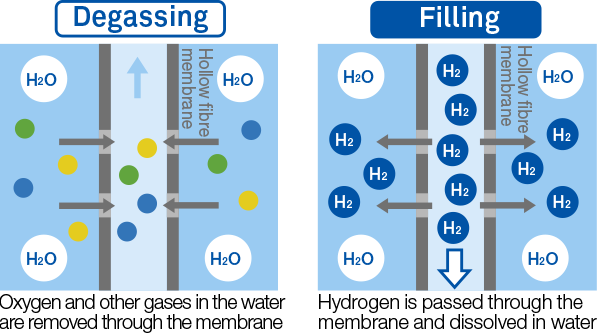
The IZUMIO manufacturing process employs a technology that makes use of a special membrane that is permeable by gases but not liquids. Through this membrane, air dissolved in water is removed from the water (through a process called “degassing” or “deaeration”), and subsequently hydrogen is dissolved in the water.
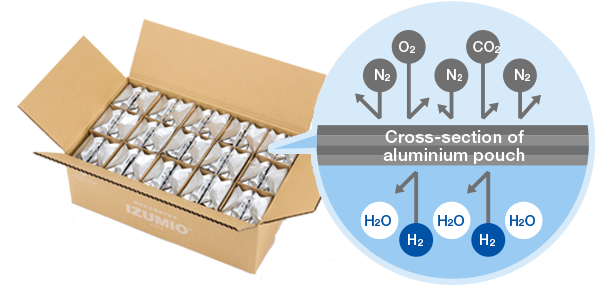
IZUMIO is packaged in a four-layered aluminium pouch that offers high shielding protection. Research and testing have been carried out to ensure that the cap on the aluminium pouch is leak-proof even when IZUMIO pouches are laid upside down in their cartons, and that the high hydrogen dissolution ratio of the product can be maintained even for long periods of storage.
The designated manufacturer for IZUMIO is located in the mountains of Tochigi prefecture, where clean air and clear water abound. The factory employs state-of-the-art facilities and is Health Supplement GMP※1-compliant and FSSC 22000※2-certified. IZUMIO is manufactured in a clean and sanitary production environment.
※1 Health Supplement GMP (Good Manufacturing Practice) refers to a standard that can be implemented on work processes and appropriate quality checks for the manufacture of quality products. (Kanuma factory & Kumamoto factory)
※2 FSSC 22000 (Food Safety System Certification) is a framework developed to ensure food safety, and is based on existing ISO Standards. As we adhere to these strict standards during production, we ensure that our products are safe for consumption. (Kanuma factory)